Which Term Describes Plants Or Animals That Carry A Foreign Gene?
GloFish are the first transgenic animals available to the American public. Only what'south the biotechnology backside them?

Effigy 1: The multicolored GloFish®.
Courtesy of world wide web.glofish.com. All rights reserved. ![]()
"Seeing is assertive with GloFish. They are absolutely stunning!" The preceding is some of the marketing material you'd read if y'all visited the GloFish website (GloFish, 2008). Dazzler may exist in the eye of the beholder, merely nearly everyone would agree that these first—and, so far, only—transgenic animals made available to the general public in the United States (except in California, pending a formal review of their potential effect on the surroundings) are a worthy conversation slice. A transgenic, or genetically modified, organism is one that has been altered through recombinant Dna engineering science, which involves either the combining of Dna from unlike genomes or the insertion of strange DNA into a genome. GloFish (Figure one) are a type of transgenic zebrafish ( Danio rerio ) that have been modified through the insertion of a green fluorescent protein (gfp) factor. Non all GloFish are green, however. Rather, at that place are several gfp gene constructs, each encoding a different colored phenotype, from fluorescent yellowish to fluorescent carmine.
Currently, GloFish are the just recombinant-DNA animal that has been approved for man "use" by the U.S. Food and Drug Administration. Their approval has raised important questions about whether, and to what extent, genetically modified animals should be made bachelor to consumers. But how were scientists able to create these engineered organisms in the first identify? Like then many genetic technologies used today, recombinant DNA technology had its origins in the late 1960s and early on 1970s. Past the 1960s, scientists had already learned that cells repair DNA breaks by reuniting, or recombining, the broken pieces. Thus, it was only a matter of time before researchers identified the raw biological ingredients necessary for recombination, figured out how those ingredients functioned together, and so tried to govern the recombining process themselves.
Early on Experiments Provide the Ground for Recombinant Organisms
Although recombinant Deoxyribonucleic acid technology outset emerged in the 1960s and 1970s, the basic principle of recombination had been discovered many years earlier. Indeed, in 1928, Frederick Griffith, an English language medical officer studying the bacteria responsible for a pneumonia epidemic in London, starting time demonstrated what he termed "genetic transformation"; here, living cells took upward genetic textile released by other cells and became phenotypically "transformed" by the new genetic data. More than a decade later, Oswald Avery repeated Griffith'southward work and isolated the transforming molecule, which turned out to be Dna. These experiments showed that DNA tin can exist transferred from one cell to another in the laboratory, thus changing the bodily genetic phenotype of an organism.
Prior to these classic experiments, the idea that the genetic cloth was a specific chemic that could be modified and transferred into cells was certainly controversial. But before the explosion in recombinant Deoxyribonucleic acid could begin, scientists would have to learn not only how to transfer DNA, but also how to isolate and modify private genes.
Fundamental Developments in Recombinant DNA Engineering
Following these early experiments, four fundamental developments helped lead to construction of the start recombinant Dna organism (Kiermer, 2007). The get-go ii developments revolved around how scientists learned to cut and paste pieces of DNA from different genomes using enzymes. The latter 2 events involved the development of techniques used to transfer foreign DNA into new host cells.
Discovering the Cut-and-Paste Enzymes
The commencement major step forward in the ability to chemically modify genes occurred when American biologist Martin Gellert and his colleagues from the National Institutes of Health purified and characterized an enzyme in Escherichia coli responsible for the actual joining, or recombining, of dissever pieces of Dna (Zimmerman et al., 1967). They chosen their find "DNA-joining enzyme," and this enzyme is at present known as Dna ligase. All living cells utilize some version of DNA ligase to "glue together" short strands of Dna during replication. Using E. coli extract, the researchers next showed that only in the presence of ligase was information technology possible to repair single-stranded breaks in λ phage DNA. (Discovered in 1950 by American microbiologist Esther Lederberg, λ phage is a virus particle that infects East. coli.) More specifically, they showed that the enzyme was able to form a iii'-5'-phosphodiester bond between the 5'-phosphate cease of the terminal nucleotide on one Deoxyribonucleic acid fragment and the 3'-OH end of the last nucleotide on an next fragment. The identification of Deoxyribonucleic acid ligase was the first of several key steps that would eventually empower scientists to attempt their own recombination experiments—experiments that involved not simply recombining the Dna of a unmarried private, but recombining DNA from unlike individuals, including different species.
A second major step forwards in cistron modification was the discovery of restriction enzymes, which cleave DNA at specific sequences. These enzymes were discovered at approximately the same time as the first DNA ligases by Swiss biologist Werner Arber and his colleagues while they were investigating a phenomenon called host-controlled restriction of bacteriophages. Bacteriophages are viruses that invade and frequently destroy their bacterial host cells; host-controlled restriction refers to the defense mechanisms that bacterial cells accept evolved to deal with these invading viruses. Arber'due south team discovered that one such mechanism is enzymatic action provided past the host jail cell. The team named the responsible enzymes "restriction enzymes" considering of the style they restrict the growth of bacteriophages. These scientists were also the starting time to demonstrate that restriction enzymes damage invading bacteriophages by cleaving the phage Dna at very specific nucleotide sequences (now known as restriction sites). The identification and characterization of restriction enzymes gave biologists the means to cut specific pieces of DNA required (or desired) for subsequent recombination.
Inserting Strange Deoxyribonucleic acid into a New Host Cell
Although Griffith and Avery had had demonstrated the ability to transfer foreign genetic material into cells decades before, this "transformation" was very inefficient, and it involved "natural" rather than manipulated Deoxyribonucleic acid. Only in the 1970s did scientists brainstorm to apply vectors to efficiently transfer genes into bacterial cells. The first such vectors were plasmids, or small DNA molecules that live naturally inside bacterial cells and replicate separately from a bacterium's chromosomal Dna.
Plasmids' utility as a DNA shuttle, or vector, was discovered by Stanford University biochemist Stanley Cohen. Scientists had already established that some bacteria had what were known as antibiotic resistance factors, or R factor-plasmids that replicated independently inside the bacterial cell. But scientists knew little about how the dissimilar R factor genes functioned. Cohen idea that if in that location were an experimental system for transforming host bacterial cells with these R-factor Deoxyribonucleic acid molecules, he and other researchers might be able to ameliorate understand R-factor biological science and effigy out exactly what information technology was nearly these plasmids that made bacteria antibiotic-resistant. He and his colleagues developed that organisation by demonstrating that calcium chloride-treated Due east. coli can be genetically transformed into antibiotic-resistant cells by the addition of purified plasmid Dna (in this case, purified R-factor Dna) to the bacteria during transformation (Cohen et al., 1972).
Recombinant Plasmids in Bacteria
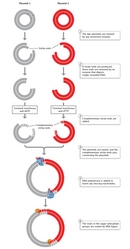
The following year, Stanley Cohen and his colleagues were also the start to construct a novel plasmid DNA from ii separate plasmid species which, when introduced into Due east. coli, possessed all the nucleotide base sequences and functions of both parent plasmids. Cohen's squad used restriction endonuclease enzymes to cleave the double-stranded DNA molecules of the two parent plasmids. The squad side by side used DNA ligase to rejoin, or recombine, the Dna fragments from the two dissimilar plasmids (Figure two). Finally, they introduced the newly recombined plasmid DNA into E. coli. The researchers were able to join two DNA fragments from completely different plasmids because, as they explained, "the nucleotide sequences cleaved are unique and cocky-complementary and then that Deoxyribonucleic acid fragments produced by i of these enzymes can associate past hydrogen-bonding with other fragments produced by the aforementioned enzyme" (Cohen et al., 1973).

The aforementioned could be said of any DNA—non just plasmids—from 2 different species. This universality—the capacity to mix and lucifer Deoxyribonucleic acid from dissimilar species, because DNA has the same structure and function in all species and because restriction and ligase enzymes cut and paste the same means in dissimilar genomes—makes recombinant DNA biological science possible.
Today, the Eastward. coli λ bacteriophage is one of the most widely used vectors used to carry recombinant Dna into bacterial cells. This virus makes an excellent vector considering virtually ane-third of its genome is considered nonessential, significant that it can be removed and replaced by strange Dna (i.e., the Deoxyribonucleic acid being inserted). As illustrated in Figure 3, the nonessential genes are removed by restriction enzymes (the specific brake enzyme EcoRI is shown in the figure), the foreign DNA inserted in their identify, and then the final recombinant DNA molecule is packaged into the virus'south protein coat and prepped for introduction into its host cell.
Vectors Used in Mammalian Cells
A fourth major step forwards in the field of recombinant DNA technology was the discovery of a vector for efficiently introducing genes into mammalian cells. Specifically, researchers learned that recombinant DNA could be introduced into the SV40 virus, a pathogen that infects both monkeys and humans. Indeed, in 1972, Stanford University researcher Paul Berg and his colleagues integrated segments of λ phage DNA, as well as a segment of Due east. coli DNA containing the galactose operon, into the SV40 genome. (The E. coli galactose operon is a cluster of genes that plays a function in galactose sugar metabolism.) The significance of their achievement was its demonstration that recombinant DNA technologies could exist applied to essentially any Dna sequences, no thing how distantly related their species of origin. In their words, these researchers "developed biochemical techniques that are more often than not applicable for joining covalently whatsoever ii Deoxyribonucleic acid molecules" (Jackson et al., 1972). While the scientists didn't actually introduce foreign Deoxyribonucleic acid into a mammalian cell in this experiment, they provided (proved) the means to practise and then.
Recombinant DNA Technology Creates Recombinant Animals
The get-go actual recombinant beast cells weren't developed until nearly a decade after the research conducted past Berg's team, and well-nigh of the early studies involved mouse cells. In 1981, for example, Franklin Costantini and Elizabeth Lacy of the University of Oxford introduced rabbit Deoxyribonucleic acid fragments containing the adult beta globin cistron into murine (mouse) germ-line cells (Costantini & Lacy, 1981). (The beta globins are a family of polypeptides that serve equally the subunits of hemoglobin molecules.) Another group of scientists had demonstrated that foreign genes could be successfully integrated into murine somatic cells, but this was the offset demonstration of their integration into germ cells. In other words, Costantini and Lacy were the start to engineer an unabridged recombinant animal (admitting with relatively low efficiency).
Interestingly, not long after the publication of his squad's 1972 study, Paul Berg led a voluntary moratorium in the scientific customs against certain types of recombinant DNA research. Clearly, scientists take always been enlightened that the ability to manipulate the genome and mix and friction match genes from different organisms, fifty-fifty different species, raises firsthand and serious questions about the potential hazards and risks of doing and then—implications even so beingness debated today.
Since these early studies, scientists have used recombinant DNA technologies to create many different types of recombinant animals, both for scientific study and for the profitable manufacturing of homo proteins. For instance, mice, goats, and cows have all been engineered to create medically valuable proteins in their milk; moreover, hormones that were in one case isolated just in pocket-size amounts from human cadavers can now be mass-produced past genetically engineered cells. In fact, the unabridged biotechnology industry is based upon the ability to add new genes to cells, plants, and animals As scientists notice important new proteins and genes, these technologies will continue to class the foundation of future generations of discoveries and medical advances.
References and Recommended Reading
Cohen, S. N., et al. Nonchromosomal antibody resistance in bacteria: Genetic transformation of Escherichia coli by R-factor DNA. Proceedings of the National University of Sciences 69, 2110–2114 (1972)
———. Construction of biologically functional bacterial plasmids in vitro. Proceedings of the National Academy of Sciences lxx, 3240–3244 (1973)
Costantini, F., & Lacy, E. Introduction of a rabbit beta-globin gene into the mouse germ line. Nature 294, 92–94 (1981) (link to commodity)
Crea, R., et al. Chemic synthesis of genes for man insulin. Proceedings of the National Academy of Sciences 75, 5765–5769 (1978)
GloFish. GloFish home folio. www.glofish.com (Accessed July 3, 2008)
Jackson, D. A., et al. Biochemical method for inserting new genetic data into DNA of simian virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proceedings of the National Academy of Sciences 69, 2904–2909 (1972)
Kiermer, V. The dawn of recombinant DNA. Nature Milestones: DNA Technologies, http://www.nature.com/milestones/miledna/full/miledna02.html (2007) (link to article)
Miller, H. I. FDA on transgenic animals—A dog's breakfast? Nature Biotechnology 26, 159–160 (2008) (link to commodity)
Zimmerman, Southward. B., et al. Enzymatic joining of Deoxyribonucleic acid strands: A novel reaction of diphosphopyridine nucleotide. Proceedings of the National Academy of Sciences 57, 1841–1848 (1967)
Source: http://www.nature.com/scitable/topicpage/recombinant-dna-technology-and-transgenic-animals-34513
Posted by: baileycoluch.blogspot.com

0 Response to "Which Term Describes Plants Or Animals That Carry A Foreign Gene?"
Post a Comment